D1: Inhibition of virus-activating host proteases
Prof. Dr. Eva Friebertshäuser
Institut für Virologie
Philipps-Universität Marburg
Hans-Meerwein-Str. 2
35043 Marburg
Tel.: +49 (0)6421-28 66019
Fax: +49 (0)6421-28 68962
E-Mail: friebertshaeuser(at)staff.uni-marburg(dot)de
Prof. Dr. Thorsten Steinmetzer
Institut für Pharmazeutische Chemie
Philipps-Universität Marburg
Marbacher Weg 10
35032 Marburg
Tel.: +49 (0)6421-28 25900
Fax: +49 (0)6421-28 25901
E-Mail: torsten.steinmetzer(at)staff.uni-marburg(dot)de
Project description:
Cleavage of viral envelope proteins by host proteases is essential for the infectivity of many human pathogenic viruses. Among others, the surface glycoproteins of highly pathogenic avian influenza viruses (e.g. H5N1), chikungunya virus or dengue, West Nile and Zika viruses are activated by furin-like serine proteases. The surface glycoproteins hemagglutinin of zoonotic H7N9 and seasonal influenza A viruses or the spike protein S of many coronaviruses (CoV) are cleaved by the membrane-bound trypsin-like serine protease TMPRSS2. Recently, we were able to show that SARS-CoV-2 S is activated by both furin and TMPRSS2. Therefore, these host proteases are promising targets for the development of novel broad-spectrum antiviral agents.
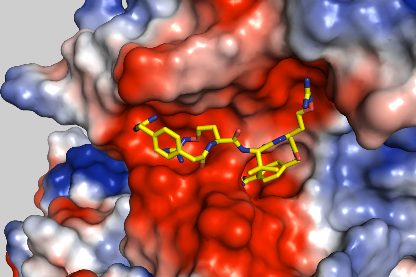
Crystal structure of furin in complex with inhibitor MI-1851

Crystal structure of TMPRSS2 superimposed with inhibitors MI-1904 (yellow) and MI-432 (orange).
Scientific goal:
Structure-based development of effective inhibitors targeting virus-activating host proteases; determination of their potency and selectivity by enzyme kinetic studies; structural characterization of their binding mode in complex with relevant host proteases; testing of their antiviral efficacy against significant human pathogenic viruses in cell cultures, tissue cultures and animal models.
DRUID Collaboration partners:
A1 Becker lab, A2 Grünweller lab, B6 Herker lab, C1 Hildt lab
References D1: 1. Böttcher et al. (2006) J Virol 80: 9896-8 3. Becker et al. (2012) J Biol Chem 287: 21992-03 4. Böttcher-Friebertshäuser et al. (2012) Vaccine 30: 7374-80 5. Ivanova et al. (2017) ChemMedChem 12: 1953-68 6. Lam van et al. (2019) ChemMedChem 14, 673-85 7. Bestle et al. (2020) LSA 3: e202000786 8. Bestle et al. (2021) J Virol 95: e0090621 9. Lam van et al. (2021) ACS Med Chem Lett 12: 426-32.


