Project area C:
C1Targets for antiviral strategies against ZIKV
Daniela Bender, Eberhard Hildt
Dr. Daniela Bender
Paul-Ehrlich-Institut
Bundesinstitut für Impfstoffe
und biomedizinische Arzneimittel
Paul-Ehrlich-Straße 51-59
63225 Langen
Tel.: +49 (0)6103-77 5411
E-Mail: Daniela.Bender(at)pei(dot)de
Prof. Dr. Eberhard Hildt
Bundesinstitut für Impfstoffe
und biomedizinische Arzneimittel
Paul-Ehrlich-Institut
Paul-Ehrlich-Straße 51-59
63225 Langen
Tel.: +49 (0)6103-77 2140
Fax: +49 (0)6103-77 1234
E-Mail: Eberhard.Hildt(at)pei(dot)de
Project description:
Zika viruses (ZIKV) are arboviruses, which belong to the flaviviridae family. During the ZIKV outbreak in Brazil in 2016, where a considerable number of microcephaly cases in newborns was associated with ZIKV infection during pregnancy. The WHO declared a public health emergency of international concern (PHEIC). At present neither a preventive vaccine or antiviral drugs are available. By inhibition of virus replication in an early phase of the viral life cycle if applicable as a temporary preventive approach, the viral load could be significantly reduced. Thereby, the spread of the virus would be impaired and the risk of an intrauterine infection would be reduced. During the first funding period target structures could be identified.
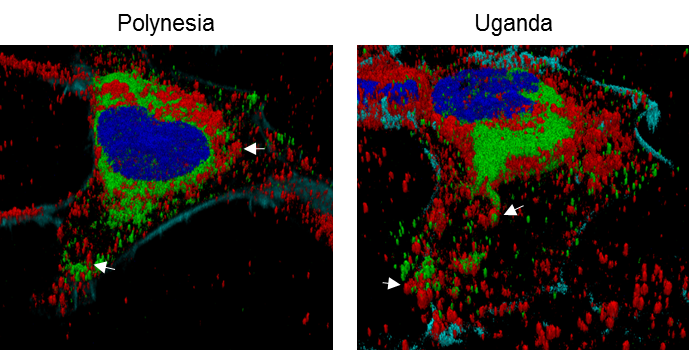
Intracellular distribution of tetherin (red) and ZIKV envelope protein (green) in cells that were infected either by the Uganda or the French Polynesia isolate.
Scientific goal:
Based on already identified targets and further targets, antiviral strategies are developed, the underlying mechanisms will be investigated and the effect on additional members of the flaviviridae family will be studied.
DRUID Collaboration partners:
A2 Grünweller lab, B1 Diderich/Kolb lab, B6P Herker lab, C2 Kempf lab, C5 Glebe/Geyer lab, D1 Steinmetzer lab, E6 Schiffmann/Laux lab
References C1: 1. Herrlein et al. (2021) J Virol. doi: 10.1128/jvi.02117-2 2. Sabino et al (2021)., J Virol. doi: 10.1128 3. Maddaluno et al., 2020 EMBO Mol Med. doi: 10.15252/emmm.201911793 4. Basic et al. 2019 Antiviral Res. doi: 10.1016/j.antiviral.2019.104644. 5. Akhras et al. (2019) Viruses doi: 10.3390/v11080748. 6. Sabino et al., (2019) doi: 10.3390/v11060524 7. Elgner et al (2018) Viruses doi: 10.3390/v10040149
C2Bartonella bacilliformis pathogenicity factors as diagnostic and therapeutic targets
Volkhard Kempf
Prof. Dr. Volkhard A. J. Kempf
Universitätsklinikum Frankfurt
Institut für Medizinische Mikrobiologie
und Krankenhaushygiene
Goethe-Universität Frankfurt/Main
Paul-Ehrlich-Str. 40
60596 Frankfurt/Main
Tel.: +49 (0)69-6301 5019
Fax: +49 (0)69-6301 83431
E-Mail: volkhard.kempf(at)kgu(dot)de
Project description
Bartonella bacilliformis is the causative agent of Carrións disease, a vector borne illness restricted to the South American Andes. The bacteria cause severe hemolytic fever with high fatality rates. The inhibition of hemolysis represents a promising therapeutic approach. Two pathogenicity factors play a crucial role in hemolysis of which at least one represents a promising drug target. Humans are the only known reservoir host for B. bacilliformis and represent the source of new outbreaks. It is therefore of particular importance for disease control to identify asymptomatic carriers. For this, an alpha version of a B. bacilliformis IgG ELISA and line blot were developed.
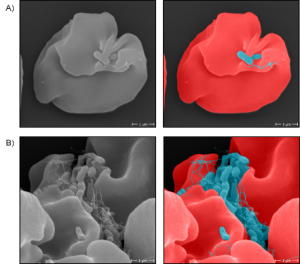
Scanning electron microscopy of infected human erythrocytes with B. bacilliformis.
B. bacilliformis NovaLisa® KIT.
Scientific goal:
The project aims to analyze the role of the two pathogenicity factors in the process of hemolysis to develop a novel, anti-virulence-based therapeutic strategy. Furthermore, the alpha version of the B. bacilliformis IgG ELISA and line blot will be evaluated in field studies in cooperation with our partners from Lima/Peru.
DRUID Collaboration partners:
A7 Przyborski lab, C1 Bender / Hildt, E7 Locker lab, Gold Standard Diagnostics (formerly NovaTec)
References C2: [1] Garcia-Quintanilla et al. (2019) Parasites&Vectors 12(1):141, [2] Riess et al. (2004) J Exp Med 200:1267-78, [3] Dichter et al. (2019) Microbiology Res Announc, DOI: 10.1128/MRA.01377-19, [4] Dichter et al. (2021) Lancet Microbe 2:e685–94.
C3Complement-interacting proteins of relapsing fever Borrelia
Peter Kraiczy
Prof. Dr. Peter Kraiczy
Goethe-Universität Frankfurt/Main
Institut für Medizinische Mikrobiologie und Krankenhaushygiene
Universitätsklinikum Frankfurt
Paul-Ehrlich-Str. 40
60596 Frankfurt am Main
Tel.: +49 (0)69-6301 7165
Fax: +49 (0)69-6301 83431
E-Mail kraiczy(at)em.uni-frankfurt(dot)de
Project description:
Borrelia recurrentis is considered as a “neglected arthropod-borne pathogen” and the causative agent of louse-borne relapsing fever. If left untreated, the case-fatality rate of this epidemic disease can exceed >40%. The hematogenous dissemination suggests that borreliae developed efficient immune evasion strategies to overcome innate immunity, in particular complement. Proteins interacting with complement are known to play a crucial role in host-pathogen interaction. Bioinformatic analyses revealed a cluster of five proteins all of which inhibit complement at different activation levels. Of importance, molecules interacting with the immune system represent promising candidates for the development of in vitro diagnostics. Two test systems (Line immunoblot and ELISA) for the diagnosis of louse-borne relapsing fever have already been developed and evaluated.
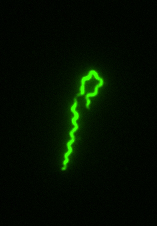
Immunofluorescene microscopy of B. recurrentis.
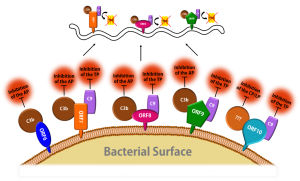
Schematic representation of the complement inactivation on the outer surface of B. recurrentis.
Scientific goal:
The focus of this project deals with the functional and structural characterization of additional complement-inhibiting proteins of Borrelia recurrentis as well as the optimization of the evaluated test systems and the development of a point-of-care antigen test for the diagnosis of relapsing fever.
DRUID Collaboration partners:
B2 Ziebuhr lab, C2 Kempf lab, C4 Steinhoff lab, D1 Steinmetzer lab, E3 Rahlfs/Przyborski, Gold Standard Diagnostics (formerly NovaTec)
References C3: [1] Cordes et al. (2005) Nat Struct Mol Biol 12:276-277; [2] Röttgerding et al. (2017) Sci Rep 7:303; [3] Nguyen et al. (2018) Front Cell Infect Microbiol. 8:23; [4] Walter et al. (2019) Front Immunol 10:2722; [5] Röttgerding and Kraiczy (2020) Front Immunol 11:1560; [6] Schmidt et al. (2021) Sci Rep 11:4964.
C4Improved diagnosis and therapy of visceral Leishmaniasis
Ulrich Steinhoff
Prof. Dr. Ulrich Steinhoff
Institut für Medizinische Mikrobiologie und Krankenhaushygiene
Philipps-Universität Marburg
Hans-Meerwein-Straße 2
35043 Marburg
Tel.: +49 (0)6421-28 66134
Fax: +49 (0)6421-58 66420
E-Mail: ulrich.steinhoff(at)staff.uni-marburg(dot)de
Project description:
A reliable diagnosis and treatment of humans and dogs (reservoir host) suffering from visceral leishmaniasis (VL) is crucial for the control of this infection. Currently available diagnostic tests based on the antibody reaction with the Leishmania kinesin protein show poor sensitivity in some endemic areas. We have developed and patented a kinesin antigen (rKLi8.3) with an improved diagnostic performance in humans and animals. This was possible by developing a kinesin antigen with an optimized kinesin structure (repeats) and sequence. Currently various test formats with the rKLi 8.3 antigen are produced and tested with sera from infected humans and dogs.
The treatment of VL is problematic in terms of effectiveness and side effects. An inhibitor (GNF-6702) that selectively inhibits the proteasome of the Kinetoplastida (T. brucei, T. cruzei and L. donovani) has been developed. GNF-6702 binds to a proteasome subunit of Kinetoplastida (beta 4 subunit) that is structurally distinct from humans. The specific bind-ing to the kinetoplast proteasome seems to be the reason for the very low toxicity in mam-malian cells. We could demonstrate that the proteasomal beta 4 subunit is conserved in all Leishmania isolates, tested to date. Thus, the proteasome is a promising therapeutic target.
Scientific goal:
In the diagnostic part of the project, various formats of a sero-diagnostic VL rapid test will be manufactured and tested in cooperation with our industrial partner. In the treatment part of the project, we validate the specificity, effectiveness and toxicity of the new, kinetoplastid-specific proteasome inhibitors in cell culture experiments of Leishmania infected macrophages.
DRUID Collaboration partners:
B1 Diederich/Kolb lab, C2 Kempf lab, D3 van Zandbergen lab
References C4: [1] Abass et al. (2013) PLoS Negl Trop Dis. 18;7; [2] Abass et al. (2015) PLoS One. 3;10; [3] Martínez Abad et al. (2017) Acta Trop. 166:133-138; [4] Pereira et al. (2020) Eur J Microbiol Immunol 27;10:165-171. [5] Khare et al. (2016) Nature 537: 229-233
C5Hit-to-lead development of selective HBV/HDV entry inhibitors
Joachim Geyer, Dieter Glebe
Prof. Dr. Dieter Glebe
Institut für Medizinische Virologie
Justus-Liebig-Universität Gießen
Schubertstraße 81
35392 Gießen
Phone: +49 (0)641-99 41246
E-Mail: dieter.glebe(at)viro.med.uni-giessen(dot)de
Prof. Dr. Joachim Geyer
Institut für Pharmakologie und Toxikologie
Justus-Liebig-Universität Gießen
Schubertstraße 81
35392 Gießen
Phone: +49 (0)641-99 38404
E-Mail: joachim.m.geyer(at)vetmed.uni-giessen(dot)de
Project description:
Infections with the Hepatitis B (HBV) and D (HDV) viruses are the main cause of hepatocellular carcinoma and liver cirrhosis as a consequence of chronic hepatitis. Although an effective prophylactic vaccine is available, therapeutic options are highly limited, in particular for HDV. A promising novel drug target to block HBV/HDV virus entry into hepatocytes is represented by the hepatic bile acid carrier NTCP (Na+/taurocholate co-transporting polypeptide) that has been identified as the bona fide hepatic receptor for HBV/HDV. So far, more than 200 compounds from different compound classes have been tested and corresponding 3D structure-activity-relationship (QSAR) models have been generated. Furthermore, a pharmacophore model for HBV/HDV entry inhibitors was established. Virtual compound libraries have already been screened with these models and further effective hits have been identified.
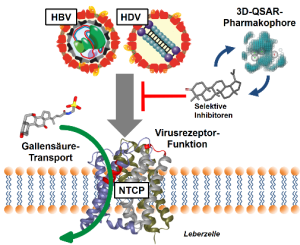
NTCP is a physiological bile acid transporter in the plasma membrane of liver cells. NTCP is also the hepatic receptor for HBV and HDV. HBV/HDV entry inhibitors should selectively block NTCP viral receptor function. 3D QSAR and pharmacophore models help identify novel NTCP inhibitors.
Scientific goal:
Development of oral and selective HBV/HDV entry inhibitors that specifically block virus binding to NTCP, without tackling its physiological bile acid transport function. Hit compounds will be further developed into lead structures by means of molecular drug design.
DRUID Collaboration partners:
B1 Diederich/Kolb, B3 Rahlfs/Kolb/van Zandbergen, D2 Pfeiffer/Zeuzem/Hildt, E3 Rahlfs/Przyborski, E6 Schiffmann/Laux
References C5:1. *Kirstgen et al. (2020) Sci Rep 10:21772 2. *Grosser et al. (2021) Front Mol Biosci 8:689757 3. *Kirstgen et al. (2021) Viruses 13:666 4. *Kirstgen et al. (2021) Viruses 13:1489.
C6The Fasciola kinome as source of new drug targets
Simone Häberlein
PD Dr. Simone Häberlein
Justus-Liebig-Universität Gießen
BFS, Institut für Parasitologie
Schubertstraße 81
35392 Gießen
Tel.: +49 (0)641-99 38476
Fax: +49 (0)641-99 38469
E-Mail: Simone.Haeberlein(at)vetmed.uni-giessen(dot)de
Project description:
Protein kinases regulate a vast variety of cellular processes and represent promising targets not only for therapy of cancer, but also infections with parasites. One common feature of both disease types is the involvement of stem cells in tissue growth. We pursue the hypothesis that inhibition of protein kinases can be employed as a new therapeutic option against the liver fluke Fasciola hepatica, a globally prevalent zoonotic and NTD-associated pathogen. The project involves three aspects: (1) bioinformatical identification of putative drug targets within the Fasciola kinome and their genetic validation, (2) identification of kinase inhibitors with activity against liver flukes; and (3) characterization of the mode of action of potent kinase inhibitors by using biochemical and imaging-based methods.
In cooperation with the Spengler lab (project E4) we established AP-MALDI mass spectrometry imaging to achieve “drug imaging” within liver fluke tissue, which allows to study the route of drug uptake, its kinetic and tissue tropism of a drug.
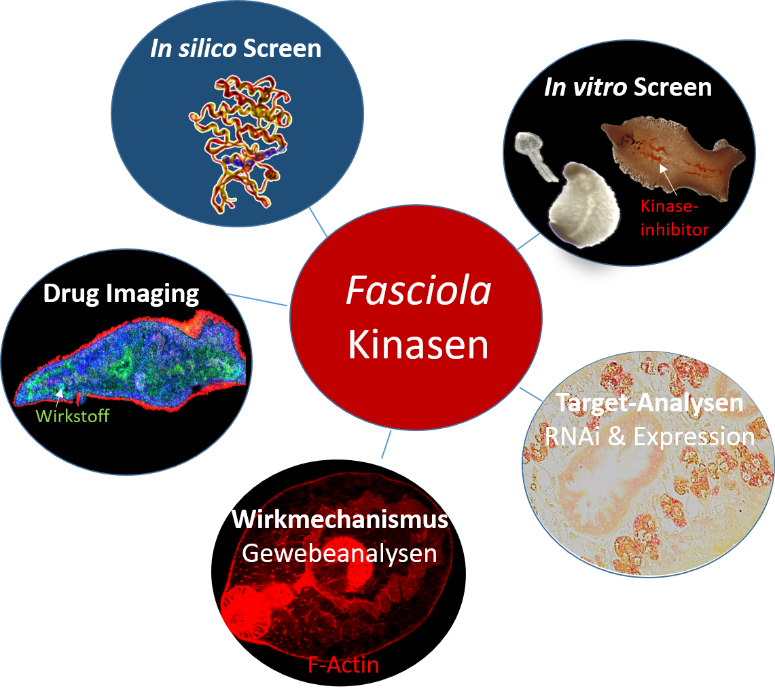
Strategy to identify protein kinase inhibitors as drug candidates against the liver fluke Fasciola hepatica. ©Simone Häberlein
Scientific goal:
This projects aims to identify protein kinase inhibitors that can serve as new drug candidates for the therapy of fasciolosis.
DRUID Collaboration partners:
A2 Grünweller lab, B4 Schlitzer lab, B5 Grevelding lab, B7 Falcone lab, E4 Spengler lab
References C6: 1. Houhou et al. (2019) Sci Rep 9:15867. 2. Li et al. (2019) Parasitol Res 118(3):881-890. 3. Morawietz et al. (2020) Front Vet Sci 7:611270. 4. Mokosch et al. Anal Bioanal Chem 413(10): 2755-2766. 5. Morawietz et al. (2022) Parasitol Res (online ahead of print) doi: 10.1007/s00436-021-07388-1








