B3: NAD(P)H-dependent metabolic pathways as targets for new anti-infective agents
Dr. Stefan Rahlfs
Biochemie und Molekularbiologie
Justus-Liebig-Universität Gießen
Heinrich-Buff-Ring 26-32
35390 Gießen
Tel.: +49 (0)641-99 39117
E-Mail: stefan.rahlfs(at)ernaehrung.uni-giessen(dot)de
Prof. Dr. Ger van Zandbergen
Abteilung Immunologie
Paul-Ehrlich-Institut
Paul-Ehrlich-Str. 51-59
63225 Langen
Tel.: +49 (0)6103-77 2005
E-Mail: Ger.Zandbergen(at)pei(dot)de
Prof. Dr. Peter Kolb
Institut für Pharmazeutische Chemie
Philipps-Universität Marburg
Marbacher Weg 8
35032 Marburg
Tel.: +49 (0)6421-28 25908
Fax: +49 (0)6421-28 26652
E-Mail: peter.kolb(at)uni-marburg(dot)de
Project description:
Cellular redox balance plays an essential role in pathogenic microorganisms. Enzymes of the NAD(P)H-dependent glutathione and thioredoxin system1,2, as well as the glucose 6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD), which significantly contribute to the NADPH and ribose 5-phosphate pool via the pentose phosphate pathway3, are centrally involved. G6PD of the malaria parasite Plasmodium falciparum and P. vivax (GluPho), present as a bifunctional enzyme, differs functionally and structurally from the human host enzyme4 and is essential for malaria parasites5. Together with the Sanford-Burnham Institute/UCSD, La Jolla, we have established a high-throughput compatible assay for PfGluPho and have screened about 400,000 compounds (i.a. NIH MLSMR Collection)6. Following structure-activity relationship studies and lead optimization, we identified the highly selective PfGluPho inhibitor SBI-750, which is active in the lower nanomolar range7. The concept could already be transferred to Leishmania; the 3D crystal structure of Leishmania donovani G6PDs and PGDs could be solved and based on this a first in silico screening of small molecules could be realized. In addition, a high-throughput compatible assay for the recombinant G6PDs and 6PGDs from Leishmania was established to screen for inhibitors at the Novartis FAST lab in Cambridge (USA).
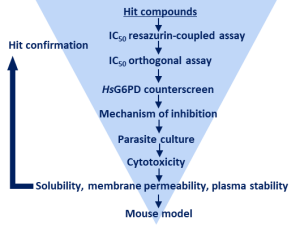
Workflow-Diagramm zur Inhibitor Identifizierung gegen G6PDs/PGDs
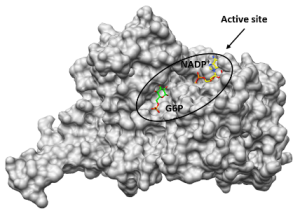
3D-Kristallstruktur LdG6PD ©Isabell Berneburg
Scientific goal:
The aim of this project is to functionally and structurally characterize the enzymes G6PD and 6PGD from Leishmania and Plasmodium as targets for drug development, and to identify and further develop inhibitors against these enzymes (in silico and HTS). The concept will also be transferred to other pathogens within the DRUID consortium, such as Schistosoma.
DRUID Collaboration partners:
B1 Diederich/Kolb; B4 Grevelding; D3 van Zandbergen; E1 Grevelding/Häberlein; E3 Rahlfs/Przyborski; E4 Spengler; E6 Schiffmann/Laux
References B3: 1. Fritz-Wolf et al. (2011) Nature Comm. 2:383* 2. Koncarevic et al. (2009) PNAS 106: 13323-8* 3. Bozdech and Ginsburg (2005) Malaria J 3:23 4. Jortzik et al. (2011) Biochem J Energy 436:641-50* 5. Allen et al. (2015) FEBS J 282:3808-23*
6. Preuss et al. (2012) J Med Chem 55:7262-72* 7. Berneburg et al. (2022) Antimicrob Agents Chemother (accepted)*
*own puplications



